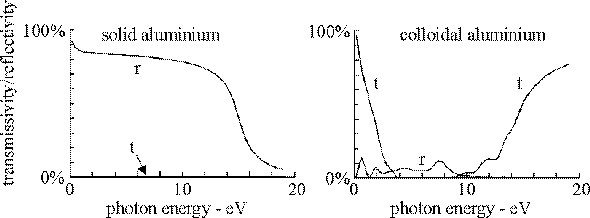

Colloidal metals are typically black: hence the use of colloidal silver in photography. In contrast solid metals are lustrous and do not absorb light. Metals are the materials of choice for high reflectivity mirrors. This contrasting behaviour is brought about by the dramatic change that colloidal structure brings to internal electromagnetic modes of the material: colloids have a high density of modes in the visible region capable of absorbing energy from incident light.
Our calculations confirm this picture: solid metal, though not highly transmitting, has a high reflectivity and relatively little of the light is absorbed. In contrast the colloid neither transmits nor reflects in the visible or near ultra violet; absorption falls off in the infra red and the colloid becomes transmitting.
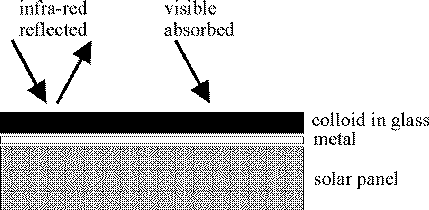
This effect is exploited in solar panel technology. Panels are covered
first with a highly reflecting surface, and next with glass containing
a suspension of metallic colloid - usually copper. Most of the sun's
energy is strongly absorbed by the colloid, but that in the infra red
region of the spectrum is lost. However this is a small price to pay
because a good reflector of infra red is also a poor emitter therefore
the relatively cool solar panel (compared to the sun) loses little
energy by radiation. At infra red wavelengths solar panels behave like
inside-out thermos flasks.